[CHINA ALUMINUM NETWORK] As mentioned earlier, aluminum fuel cells produce electricity by "burning" aluminum. The "combustion" mentioned here is actually aluminum dissolved in an alkaline solution (electrolyte) to change aluminum ions (Al→Al+3+3e), emits three electrons (3e), is an anode, and electrons “run†toward the cathode. A simple electrochemical reaction with oxygen in the air releases the process of energy generation. We can think of a fuel cell as a “factory†that will transport fuel (aluminum) in. At the same time, it will output the generated electricity. If aluminum is present, it will generate electricity continuously. This is the fundamental difference between fuel cells and traditional batteries. Although they all rely on the principle of electrochemistry to work. Aluminum fuel cells consist of an aluminum anode, an air cathode, and an electrolyte. The electrolyte is generally an alkaline solution. Aluminum fuel cells also require the catalytic action of the catalyst, a chemical power source that generates a chemical reaction under the catalytic action of the catalyst.
For primary batteries such as aluminum fuel cells, aluminum releases electrons and becomes ions. Aluminum is the anode and air is the cathode. After the anode and cathode are connected, electrons run from the cathode to the anode. For ordinary batteries, there is positive The difference between negative and negative current flows from the positive electrode to the negative electrode. Aluminum fuel cells are the inverse process of aluminum electrolysis.
The operation of the aluminum fuel cell system, air entering from the left, filtering and cleaning and flowing into the cathode of the aluminum fuel cell stack (aluminum empty battery stack), is based on the previous step, that is, sufficient aluminum anode plates and air cathodes for supplying oxygen are prepared. The plate, to supply enough oxygen; the second step is the electrochemical reaction, once the electrochemical reaction occurs, the current will be generated, the current size is closely related to the speed of the electrochemical reaction, the faster the speed, the more current is generated, for which we With the aid of a catalyst and a fine reaction zone design to increase the reaction rate; the third step is the transmission of ions or electrons, the electrochemical reactions that take place during the process will generate or consume ions and electrons, and the ions produced by the aluminum electrode are on the other side of the air (oxygen) electrode. Consumed, electrons are also the same, in order to maintain the charge balance, they must be transported from the produced area to the area they consume. Once they are connected by wires, electrons flow from one electrode to the other, but the flow of ions is better than Electronic is much more difficult because it is much larger than electrons and it weighs a lot. It must rely on electrolytes to transport aluminum. Cells as electrolyte is an alkaline solution. In the fourth step, the product is discharged. In addition to generating electricity, at least one type of reactant is generated in any kind of fuel cell. Even a simpler hydrogen-oxygen fuel cell generates water, and the aluminum fuel cell generates Al(OH)3. Must be discharged from the battery in time, otherwise it will accumulate in the battery over time, hindering the reaction of aluminum and oxygen, compared to the final battery will "suffocate" and die.
There are five elements in the operation of aluminum fuel cells: anodes, cathodes, electrolytes, catalysts, and reaction products, which will be introduced in the next article. Now, aluminum fuel cells have formed a very good closed circuit.
Aluminum fuel cell technology can be roughly summarized as: is a direct electrochemical energy conversion device, through the electrochemical reaction directly from a form (chemical energy) into another energy form - electrical energy; aluminum fuel cell is not Like a normal battery, it will not run out, but it will be more like a "factory". As long as there is fuel supply, it will produce electricity continuously. The aluminum fuel cell must have two electrodes, yin and yang, divided by two electrolytes; aluminum fuel. The power of a battery depends on its size. The energy depends on its fuel storage. The electrochemical system must contain two pairs of semi-reactions: oxidation reaction and reduction reaction. The oxidation reaction releases electrons. The reduction reaction consumes electrons. The oxidation reaction occurs in Anodized aluminum electrode, the reduction reaction occurs in the cathode electrode oxygen; the four main steps produced in the aluminum fuel cell are: fuel aluminum and oxygen two reactants transport, electrochemical reaction, ion and electron conduction, product Al(OH) 3 Exclude; Aluminium fuel cell performance is evaluated using a current-voltage curve, which represents the output voltage of an aluminum fuel cell at a given current load; Loss, the actual fuel cell performance is better than aluminum over the fuel cell is poor, the main types of losses: activation losses, ohmic (resistive) loss, loss of concentration.
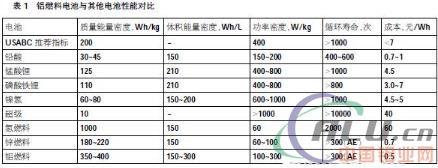
The performance comparison between aluminum fuel cells and other batteries is shown in Table 1. From the data in the table, the overall performance of aluminum fuel cells is significantly better than that of other batteries.
Among the nine quantitative indicators introduced in Table 1, the more important are the energy density and power density, which are now explained in order to deepen the understanding of aluminum fuel cells. Energy is defined as the ability to do work, commonly used in units of J (Joules) or Cal (calories or cards); power is defined as the rate at which energy is consumed or produced, and its typical unit is W (watts or watts), which represents every second. The energy consumed or generated, 1W = 1 J/s, it can be seen that energy = power × time.
Volume power density refers to the amount of power that can be provided by a device per unit volume (cm3, m3, L), and its typical unit is W/cm3 or kW/m3. The mass power density or specific power refers to the amount of power provided per unit mass of the device, and its typical unit is W/g or kW/kg.
Bias Airway buffing wheel, also called as airway buffs, airflow buffs, Ventilated buffs. The special air folded structure brings bias airway buffing polishing wheel strong cutting force, high brightness, fine finishing, can cool the workpiece surface at a very short time. Make airway buffing polishing wheel widely use on metal and non metal products polishing, such as wood, stainless steel, aluminum, copper etc. Involved in variety of industries: furniture, automobile spare part, hardware, building materials,etc.
Airway buffs are a type of buffing and polishing wheel that is made up of multiple plys of cotton Cloth attached to a steel center plate. Each cloth layer of these 16-ply wheels is attached to the steel plate in a way that forces the cotton to form pleats.
High-speed buffing and polishing wheels can create a lot of heat from the constant friction of the wheel, surface being buffed, and Polishing Compound grinding at the same time. The fold are designed to increase airflow so that you can buff and polish at a lower temperature. This allows you to work continuously longer than traditional polishing wheels, extends the overall serving life of the airway buff, and lowers the chance of causing damage or burn to the object you are polishing.
The different colors of airway buffs are not there to let you work with your favorite color. Each of these colors has a different firmness/softness of the cloth pieces which are meant for different steps in the polishing process. You should also consider pairing each color buffing wheel with the appropriate color buffing compounds.
Cotton Polishing Buff Wheel,Sisal Buffing Wheel,Flap Buffing Wheel ,Non-woven Wheel Polishing
Jiangmen Gude Polishing Equipment Co., Ltd , https://www.kokipolishing.com