HC Plastics News: 2017 is the 50th anniversary of the birth of DuPont Tyvek. On this occasion, DuPont Tyvek launched the world's newest Tyvek 40L medical packaging material at MEDTEC2017 (the 13th International Medical Device Design and Manufacturing Technology Exhibition) on September 20, 2017. This new specification of Tyvek materials for medical packaging applications is designed to provide a more cost effective packaging option for lighter weight and lower risk medical devices.
DuPont Tyvek/Typar Global Business Director Christian Marx, Tyvek Medical Packaging Global Business Director Margaret Pyers, Tyvek Medical Packaging Asia Pacific Marketing Manager Kevin Wu, Tyvek Medical Packaging Global Sales Director Leslie Love, Tyvek Asia Pacific Sales Director Suo longNi and Tyvek/Typar Worldwide Lori Gettelfinger, Director of Brand and Market Communications, attended the lighting ceremony and officially released the new products that the industry has been waiting for for a long time. It is reported that the Tyvek 40L will be officially launched in China first, and plans to be listed in other Asian countries, Europe and North America.
Since 2015, China's CFDA has initiated a series of reforms to launch strict quality inspections for the pharmaceutical and equipment industries, and to improve the quality requirements of the industry. This move has also objectively promoted the selection of a large number of domestic enterprises. A safe and reliable manufacturer of branded packaging materials.
“As a new addition to the Tyvek family of medical packaging products, the Tyvek 40L interprets our philosophy from beginning to end – making medical packaging easier,†said Marveet Pyers, global business director for Tyvek Medical Packaging, “Tyvek 40L, a groundbreaking product, Allows users to package lighter weight and lower risk medical devices with more suitable materials, especially in emerging Asian markets. We are committed to meeting market needs with innovative products and will continue to uphold this brand tradition in the future. ."
Over the years, Tyvek has become a recognized example of the medical packaging industry with its superior protective properties, which have been used in almost all forms of aseptic medical packaging and related medicine.
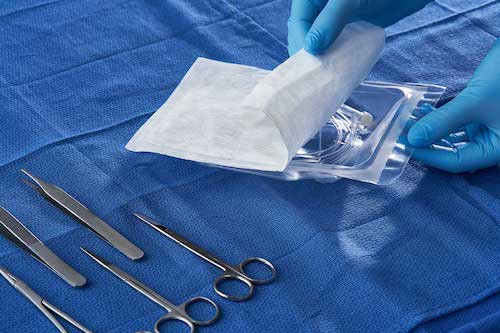
The Tyvek 40L continues the many advantages of the Tyvek range, such as low chipping, clean peeling, breathability and excellent microbial barrier properties even in harsh environments; during handling, transport and storage (even in high humidity) Provides good physical protection in cold or cold conditions; and adapts to all major sterilization methods. It is these advantages that have made Tyvek materials the industry benchmark for medical packaging since 1972. This new product is lighter, more breathable and performs well on ethylene oxide (EO) and gamma radiation sterilization. The unique texture is presented through original technology, and the unique appearance also gives the package more design and recognition. Compared to reinforced medical paper, the Tyvek 40L also exhibits superior physical strength and breathability. Relying on its low basis weight, Tyvek 40L greatly reduces the use of packaging materials and promotes sustainable development.
In addition to providing a range of high performance microbial barrier products for the medical packaging industry, such as Tyvek 1073B, Tyvek 1059B, Tyvek 2FS and the new Tyvek 40L, the DuPont Medical and Pharmaceutical Packaging team is also committed to providing technical support to medical device manufacturers worldwide. Explore industry standards, compliance information, technology and product quality issues. Team members actively participate in technical activities carried out by major standardization bodies and industry associations, contributing to industry standards and technology development, such as the International Organization for Standardization (ISO), the European Committee for Standardization (CEN), the American Association for the Advancement of Medical Devices (AAMI), And the China National Standardization Administration (SAC). 
Editor in charge: Ye Dan
Led Par Light,Par16 Led Bulb,Par56 Led Bulb,Par 46 Led Bulb
Big Dipper Laser Science And Technology Co., Ltd , https://www.bigdipper-laser.com