The principle of electrophoresis is similar to that of electroplating. The principle is that in the electrolyte composed of conductive water-soluble or water-emulsified coating, the Other electrode in the workpiece and the electrolyte are respectively connected to the two ends of the DC power source to form an electrolytic circuit. The dissociated cations move toward the cathode under the action of an electric field, and the anions move toward the anode. These charged resin ions, along with the adsorbed pigment particles, electrophores on the surface of the workpiece and lose charge to form a wet coating.
Electrophoresis classification The current electrophoresis coating is equipped with both anodic electrophoresis and cathodic electrophoresis.
The water-soluble resin used for anodic electrophoresis is an anionic compound. In water, the water-soluble resin (amine carboxylate) dissolves into an ionic form. If a direct current electric field is applied, the potential difference generated between the two poles causes the ions to move toward the two poles. The anion moves toward the anode and deposits on the surface of the anode, releasing electrons; the cation moves toward the cathode and is reduced to an amine (or ammonia) at the cathode to obtain electrons.
The water-soluble resin used for cathodic electrophoresis is a cationic compound. After neutralization with an organic acid, it dissolves into an ionic form in water. After passing through a direct current electric field, the ions move in a direction, the cation moves toward the cathode, and electrons are released on the surface of the cathode to be oxidized to an acid.Electrophoresis development process
So far, the coatings used in electrophoretic coating have been through 6 generations, and the first and second generation electrophoretic coatings are anodic electrophoretic coatings. The various generations of paints are now briefly described below.
The first generation is a low voltage, low throwing anodic electrophoretic coating. It is mainly used for interior painting of automobile bodies, and an auxiliary cathode must be provided. Its salt spray resistance is poor, and it is represented by maleic anhydride oil, phenolic acid, epoxy ester, etc. within 100 hours.
The second generation is a high voltage, high throwing anodic electrophoretic coating. The auxiliary cathode can no longer be placed when painting the body of the car. The salt spray resistance is greatly improved, and it can reach more than 240h (electrophoretic coating on the phosphating plate), and is represented by polybutadiene resin.
The third generation is a low voltage, low throwing power, low pH cathodic electrophoretic coating. The pH of the electrophoresis bath is 3 to 5, the acidity is strong, and the tank body is quickly corroded. However, the corrosion resistance of the automobile body coating after electrophoresis is improved, and it can reach 360 to 500 hours.
The fourth generation is a high voltage, high pH, ​​high throwing capacity cathodic electrophoretic coating. The pH of the electrophoresis bath is about 6.0, and the salt spray resistance test on the phosphating plate can reach 720h or more. It is still the mainstream of cathodic electrophoresis in various countries.
The fifth generation is a thick film cathodic electrophoretic coating. Mainly to improve the corrosion resistance of the sharp edges of the coated workpiece and to simplify the coating process. The film thickness is 30-35μm, and the salt spray resistance can reach about 1000h.
The sixth generation is a high pH, ​​high throwing power, lead-free, environmentally friendly cathodic electrophoretic coating. The remarkable environmental characteristics of this generation of coatings are reduced curing temperatures and energy and resources.
The specific performance comparison is shown in Table 3-20.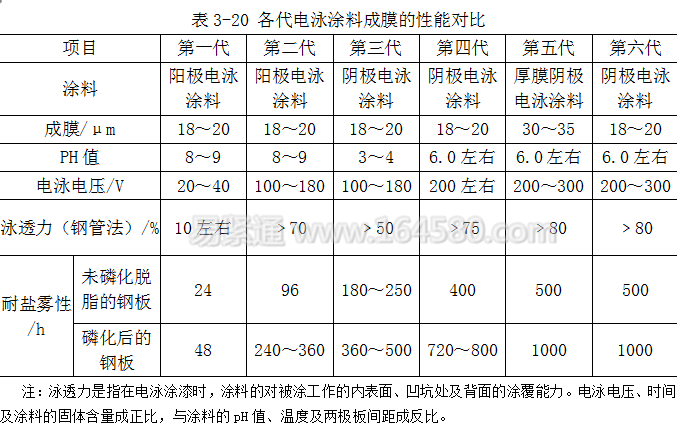
Electrophoresis characteristics
The advantages of electrophoretic coating are:
(1) The working environment is good. The solvent in the electrophoretic coating electrolyte is water, no flammable or explosive problem, and does not pollute the air.
(2) High production efficiency. Compared with other coating methods, electrophoretic coating has the highest production efficiency. The workpiece is immersed in the electrolyte and can be electrophoresed within a few minutes. It is suitable for mass production and easy to automate production.
(3) Saving raw materials. The material utilization rate of electrophoretic coating is generally above 85%, which is 40% lower than painting.
(4) The coating quality is good. The surface of the electrophoretic coating is uniform, the adhesion to the workpiece is good, the paint film is tight, and there are no defects such as flow marks and foaming.
But electrophoresis also has some drawbacks, such as:
(1) The equipment is complex and the investment is large. In addition to the electrophoresis tank, it also needs auxiliary equipment, ultrafiltration equipment and pure water equipment, special DC power supply, drying equipment, and wastewater treatment equipment.
(2) There are few varieties of paint. Currently, electrophoretic coatings are limited to water-soluble paints and water-based emulsion paints; the color is limited to dark primers or single-layer underside paints. The reason for this is that during the electrophoresis process (such as anodic electrophoretic deposition), the ionized iron ions and resin anions are neutralized and deposited on the workpiece to be yellowish brown.
(3) The electrophoretic coating needs to be baked at 150 ° C for 1 h, which consumes a large amount of energy.Electrophoresis Electrophoretic coating is a very complex electrochemical process, mainly including four simultaneous processes of electrophoresis, electrolysis, electrodeposition and electroosmosis.
(1) Electrophoresis. Under the action of an applied electric field, the charged particles (colloidal resin particles) in the solution move toward the oppositely charged electrode plates, and the uncharged pigments are adsorbed on the charged colloidal resin particles and electrophoresed.
(2) Electrodeposition. Under the action of an applied electric field, the charged resin particles are electrophoresed to the anode (or cathode), and electrons are released (or obtained) and deposited on the surface of the anode (or cathode) to form a water-insoluble coating.
(3) Electroosmosis. Electroosmosis is the inverse of electrophoresis. Its main function is to dehydrate the electrodeposited coating. When the colloidal resin particles are deposited on the surface of the anode, a medium such as water originally adsorbed on the anode plate passes through the coating into the solution under the action of the internal osmotic force.
(4) Electrolysis. Under the action of an applied electric field, an electric current passes through the electrolyte solution, which electrolyzes water, releases hydrogen gas at the cathode, and emits oxygen at the anode. Therefore, in the electrophoretic coating process, the voltage should be appropriately reduced to eliminate the influence of hydrogen and oxygen generated by the electrolyzed water on the coating quality.
In the above four reaction processes, electrophoresis is the main process of moving charged particles to the workpiece. Electrodeposition and electroosmosis are adhesion to the coating particles on the workpiece, while electrolysis mainly causes side effects, and electrolysis will affect the quality of the paint film.The electrophoresis process currently uses a lot of electrophoretic coating process: pre-treatment (de-oiling→water washing→rust removal→water washing→phosphating→water washing→passivation)→electrophoretic coating→pure water cleaning→baking film formation. In addition, the pretreatment process can also take the steps of sand blasting→water washing.
(1) Degreasing. Generally, the chemical degreasing method is used, the temperature is 50 to 70 ° C, and the time is 15-20 min.
(2) Washing. At room temperature, the washing and degreasing liquid is used as a standard.
(3) Derusting. Use sulfuric acid, hydrochloric acid, phosphoric acid or special rust remover to clean according to specific regulations.
(4) Washing. At room temperature, the descaling solution is used as a standard.
(5) Phosphating. Including the two steps of surface adjustment and phosphating, a commercially available phosphating solution can be used, and the phosphating operation is carried out according to the instructions for use.
(6) Passivation. Use a drug compatible with the phosphating solution (provided by the phosphating solution manufacturer), 1 to 2 minutes at room temperature.
The above process can be replaced by a process of sandblasting and water washing.
(7) Electrophoretic coating. Special attention should be paid to the fact that the workpiece enters and exits the electrophoresis tank to be powered off.
(8) Pure water cleaning. Wash with treated pure water.
(9) Drying. The cleaned workpiece is placed in an oven for drying.Factors affecting electrophoresis
The factors affecting the electrophoresis effect are more complicated, mainly including:
(1) The concentration of the coating in the electrolyte. In general, the effective solid content of the electrophoretic electrolyte should be kept at 10% to 15% (mass fraction). During the production process, the composition of the electrolyte will change due to the continuous consumption of the active ingredients. It should be monitored at any time, and the coating should be added regularly to keep the content of effective solid components in the electrolyte stable.
In addition, distilled water or deionized water is used in the preparation of the electrolyte to avoid interference with other coating properties such as adhesion of the coating ions to the coating during electrophoresis.
(2) The temperature of the electrolyte. It is preferred to control the temperature in the electrolyte during electrophoresis in the range of 20 to 30 °C. If the temperature is too high, the evaporation of additives and water in the electrolyte will be accelerated, and the electrophoretic coating will be thick, rough and sag. The temperature is too low, the water solubility of the solid components is reduced, and the deposition on the surface of the workpiece is reduced. The layer is easily thinned, dull, and even partially uncoated. Therefore, the temperature of the electrophoresis tank should be accurately controlled.
(3) The pH of the electrolyte. Anode electrophoresis, the electrolyte contains ammonia or alkali to form a salt, and the solution is alkaline. During electrophoresis, due to the continuous consumption of the negatively charged effective solid component, ammonia (or amine) is continuously generated in the vicinity of the cathode, and the pH of the electrolyte is continuously increased. When the pH value is too high, the electrophoretic coating is thin and dull, and there is no electrophoretic coating on the pit. When the workpiece is taken out from the electrophoresis tank, the coating is easily dissolved again to cause poor adhesion; when the pH is too low, The coating composition in the electrolyte is insoluble in water, and sag is likely to occur during the subsequent washing process. Therefore, strict control of the pH value of the electrolyte plays an important role in the speed of electrophoresis, penetration, coating performance, and coating utilization. The adjustment methods mainly include: adding a low-amine or amine-free paint, using an ion exchange resin, a cathode cover diaphragm method, an electrodialysis method, and the like.
Cathodic electrophoresis, the electrolyte is acidic. If the pH value is too high, the dispersion stability of the coating is lowered; if the pH value is too low and the acidity is enhanced, the corrosion of the electrolytic cell and its pipeline will be aggravated, and the throwing power will decrease.
(4) Conductivity in the electrolyte. Generally, when the concentration, temperature and pH of the electrolyte remain stable, the conductivity is too high, indicating that the concentration of electrolyte (in this case, impurity ions) in the electrolyte increases, the coating is easily deteriorated and settled, and the surface of the coating is rough. The rust prevention ability is reduced, and even the coating cannot be formed; if the conductivity is too low, the conductivity of the electrolyte is poor, the electrophoresis time is prolonged, the efficiency is lowered, and the formed coating is thin. Therefore, in the electrophoresis process, the most common case is the increase in conductivity, and the conductivity should be strictly controlled.
(5) Electrophoresis voltage. During the electrophoresis process, the voltage is too high, which can speed up the electrophoresis speed, but the coating film is thick, rough, poor adhesion, easy to sag, orange peel, etc.; the voltage is too low, the electrophoresis speed is reduced, and the formed coating film is thin. It has poor corrosion resistance and is prone to local uncoated phenomenon.
(6) Electrophoresis time. When the above other conditions are normal, the time of electrophoresis should also be strictly controlled. If the time is too long, the coating film may be thick, rough and poorly adhered; the time is too short, the coating is thin and dull, and there is no electrophoretic coating on the pit. The coating has reduced corrosion resistance.
(7) "L" effect. The "L" effect means that when the "L" shaped workpiece is electrophoretically coated, due to the difference between the vertical surface and the horizontal plane, defects such as rough coating, poor particle size, and poor glossiness are likely to occur on the horizontal surface, and attention should be paid. During electrophoresis, the electrolyte should be stirred frequently to lift the workpiece to maintain the overall uniformity of the solution.
kaiping aida sanitary ware technology co.,ltd , https://www.kpaidafaucets-jm.com