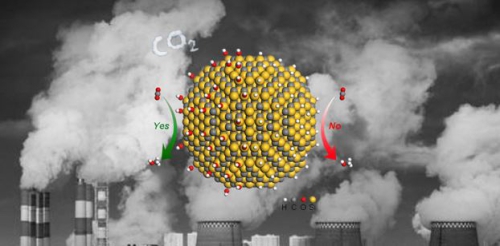
SiC Catalytic CO2 Hydrogenation Reaction
Recently, Professor Zeng Jie's group at the Hefei National Institute for Physical Sciences at the Microscale Research Institute and the School of Chemistry and Materials Science at the University of Science and Technology of China has used the silicon carbide system as a research object and found that the hydrophilicity and hydrophobicity play an important role in the catalytic reaction process, and from the atomic scale The "source" of this effect is explained: Hydrophilic silicon carbide quantum dots are rich in hydroxyl groups on the surface and can effectively promote the activation of carbon dioxide molecules. The research results were published online on Chem by Molecular-Level Insight into How Hydroxyl Groups Boost Catalytic Activity in CO2 Hydrogenation into Methanol. The co-first authors of the dissertation were PhD students Peng Yaohan, Ph.D. Wang Liangbing and Special Associate Researcher Luo Qiquan.
Catalytic reactions occur on the surface of the catalyst and can generally regulate the properties of the catalyst surface to increase the activity, selectivity, and stability of the catalytic reaction. Hydrophobicity is an important surface property parameter. In the past, people's understanding of the nature of hydrophilicity and hydrophobicity basically stayed in the enrichment of substrate molecules. For example, the hydrophilic catalyst surface easily adsorbs alcohols and other species, while the hydrophobic surface is easily adsorbed. Ester ketones and other species. However, this understanding is more macroscopic, so it reveals that the nature of the catalytic reaction on the surface of the catalyst affects the nature of the catalytic reaction from the atomic scale, which is of important guiding significance for the design of high-efficiency catalysts.
The researchers compared the activity of carbon dioxide hydrogenation in commercial silicon carbide and quantum dot silicon carbide. It was found that the hydrophilic quantum dot silicon carbide has a mass activity lower than that of hydrophobic commercial silicon carbide under the conditions of 32 atm and 150oC. Three orders of magnitude higher. The apparent activation energy of QD silicon carbide is (48.6 kJ mol-1), which is about half that of commercial silicon carbide (94.7 kJ mol-1). Using in situ synchrotron radiation X-ray photoelectron spectroscopy and near-edge X-ray absorption spectroscopy, the researchers found that the hydrophilic quantum dot silicon carbide surface is rich in hydroxyl groups, and the H atoms on the hydroxyl group can directly interact with carbon dioxide to form HCOO. * Intermediate species, which are directly involved in the catalytic reaction process. This special reaction path reduces the activation energy of HCOO* formation and thus promotes the activation of carbon dioxide. Based on this understanding, the researchers built a series of surface-enriched catalysts that are more than an order of magnitude more active in CO2 hydrogenation than their hydroxyl-free counterparts. The understanding of the role of hydrophilicity and hydrophobicity in the hydrogenation of CO2 exceeded the traditional understanding of hydrophilicity and hydrophobicity and opened up new ideas for the future to find more efficient CO2 hydrogenation catalysts.
The research work was supported by the Frontier Science Key Research Project of the Chinese Academy of Sciences, the National Major Scientific Research Plan, and the National Natural Science Foundation.
Stevia Blends,Stevia Erythritol Blend,Coffee-Used Stevia Blends,Sugar Substitute Blends
JINING USP INTERNATIONAL CO.,LTD. , https://www.uspintl.com