Researchers at Rice University in the United States have found a way to simplify the manufacturing of solar cells by using the top electrode as a catalyst to turn pure silicon into more valuable "black silicon."
Black silicon has a highly textured surface with nanoscale protrusions or pores that are smaller than the wavelength of light, allowing it to efficiently collect light from any angle at any time of the day. Andrew Barron, professor of chemistry at Rice University, said that he and the research team have been adjusting for the production of black silicon for a long time. Now, with the advancement of process technology, it is expected to help further commercialization.
Barron said that this study was mainly conducted by Yen-Tien Lu, a postdoctoral research fellow at Rice University, and has now made two major discoveries. "First of all, it's always a good thing to be able to reduce process steps," Barron explained. "Second, this is the first time that metal has been used as a catalyst for reactions other than a few millimeters away."
The method of using the metal layer as the top electrode is generally suitable for the final step of solar cell manufacturing. This new method, called "contact-assisted chemical etching," can be used to set gold fine wires as electrodes in the initial process, without having to remove used catalyst particles.
The researchers found that etching with chemical bath deposition also occurred outside the set metal lines. Barron said that the existence of this distance seems to be related to the semiconductor properties of silicon.
"Yen-Tien reacted with the gold top contact and added silver or gold as a catalyst, and finally got a good result," Barron explained. "Later I said, 'Now let's do an experiment without catalyst "Suddenly, we got black silicon - but it can only be etched at a certain distance away from the contacts. No matter how we experiment, it will appear that distance."
Electron microscopy images show the rapid production of fine protrusions and pores that absorb light on the surface of the chip used for solar cells. Rice University researchers have developed a dual-tasked gold electrode in the black silicon process that accelerates surface etching as a catalyst.
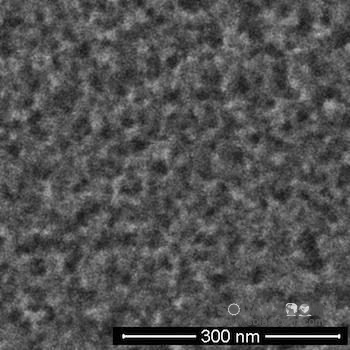
Electron microscopy images show rapid fabrication of fine protrusions and pores that absorb light on the surface of the chip used for solar cells. Rice University researchers have developed a dual-tasked gold electrode in the black silicon process that accelerates surface etching as a catalyst.
Source:Rice University/Barron Research Group
"This result tells us that the electrochemical reaction takes place at the metal contacts and maintains a certain distance from the silicon crystals," Baron said. "This distance depends on the charge-carrying properties and conductivity of the silicon crystals. Sometimes, the conductivity is insufficient." It cannot carry the charge to more distant distances."
A very thin layer of gold wire covering the titanium has proven to be an effective electrode as a catalyst. Barron emphasized that "the key is to be etched deep enough to avoid the reflection of sunlight, and if not deep enough, it may eventually lead to a battery short circuit."
"Metal contacts are usually placed last," Barron said. "There are still many pending issues in the process, such as whether to place contacts as early as possible and whether to use it for chemical reactions in later processes." â€
Street Light,Solar Parking Lot Lights,Street Lamp,Led Street Lamp
Zhongshan Tiger Lighting Co.,Ltd. , https://www.tigerstreetlight.com